
隨著人們環保意識的增強,電催化作為一種潔凈的催化過程越來越受到重視,被廣泛應用于有機電合成、燃料電池、電催化降解、二氧化碳和氮氣的電催化還原等領域。開發研制新型的高效電催化材料是電催化應用研究中的核心技術。納米材料引入電催化研究中,給新型電催化材料的開發注入了新的活力。
納米電催化材料為電催化的研究開辟了新天地。作為一種新型的電催化劑,它不僅能極大改善電極的電催化性能,而且為深入研究電催化機理提供了可能。尺寸、形貌、成分和結構等因素都會對電催化劑的催化性能產生重要影響。那么,當前電催化領域的研究處于什么狀態?電催化領域的前沿問題和關鍵問題有哪些?電催化領域未來的發展方向是什么?電催化何時能夠得到大規模實際應用?
有鑒于此,催化劑編輯部開辟了電催化周刊欄目,精選每周電催化領域中頂級期刊、電催化大牛的成果和重大研究進展,通過簡報的形式帶大家關注最新的電催化前沿動態,聚焦那些顛覆性突破。
這是電催化周刊的第2期,催化計編輯部對上周有關電催化的突出研究成果進行歸納總結,供大家學習交流。(點擊圖片,可查看詳情)

Part 1單原子催化
Part 2 HER/OER/ORR
Part 3電催化其他進展




Part 4 電催化綜述


參考文獻:
1. Kaipeng Liu, et al. Strongmetal-support interaction promoted scalable production of thermally stablesingle-atom catalysts. Nat Commun ,2020
DOI:10.1038/s41467-020-14984-9
https://doi.org/10.1038/s41467-020-14984-9
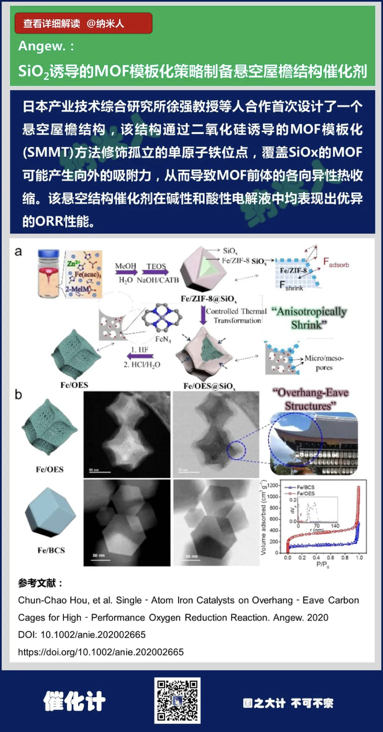
2. Chun-Chao Hou, et al. Single‐Atom Iron Catalysts on Overhang‐Eave CarbonCages for High‐Performance Oxygen Reduction Reaction.Angew. 2020
DOI: 10.1002/anie.202002665
https://doi.org/10.1002/anie.202002665
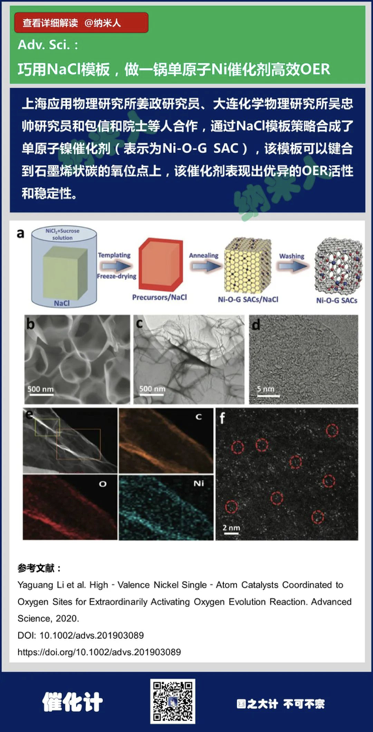
3. Yaguang Li et al. High‐Valence Nickel Single‐Atom CatalystsCoordinated to Oxygen Sites for Extraordinarily Activating Oxygen EvolutionReaction. Advanced Science, 2020.
DOI: 10.1002/advs.201903089
https://doi.org/10.1002/advs.201903089
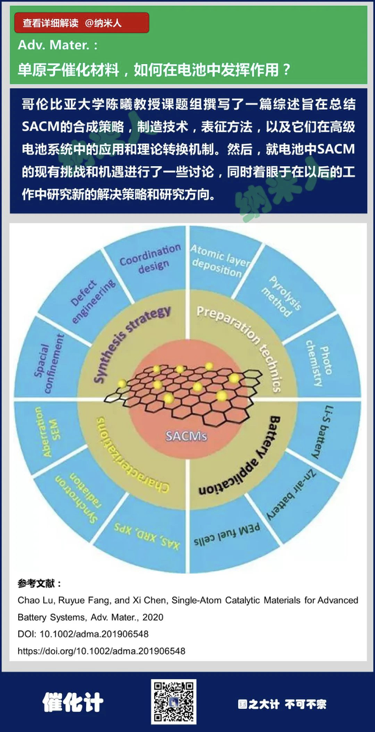
4. Chao Lu, Ruyue Fang, and XiChen, Single-Atom Catalytic Materials for Advanced Battery Systems, Adv.Mater., 2020
DOI: 10.1002/adma.201906548
https://doi.org/10.1002/adma.201906548
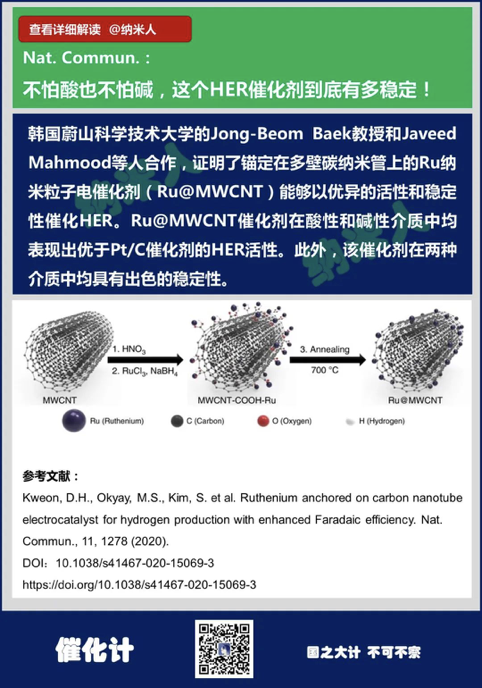
5. Kweon, D.H., Okyay, M.S.,Kim, S. et al. Ruthenium anchored on carbon nanotube electrocatalyst forhydrogen production with enhanced Faradaic efficiency. Nat. Commun., 11, 1278(2020).
DOI:10.1038/s41467-020-15069-3
https://doi.org/10.1038/s41467-020-15069-3
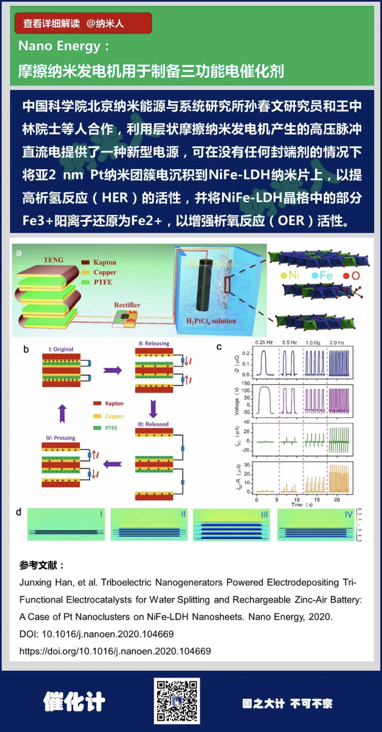
6. Junxing Han, et al.Triboelectric Nanogenerators Powered Electrodepositing Tri-FunctionalElectrocatalysts for Water Splitting and Rechargeable Zinc-Air Battery: A Caseof Pt Nanoclusters on NiFe-LDH Nanosheets. Nano Energy, 2020.
DOI:10.1016/j.nanoen.2020.104669
https://doi.org/10.1016/j.nanoen.2020.104669
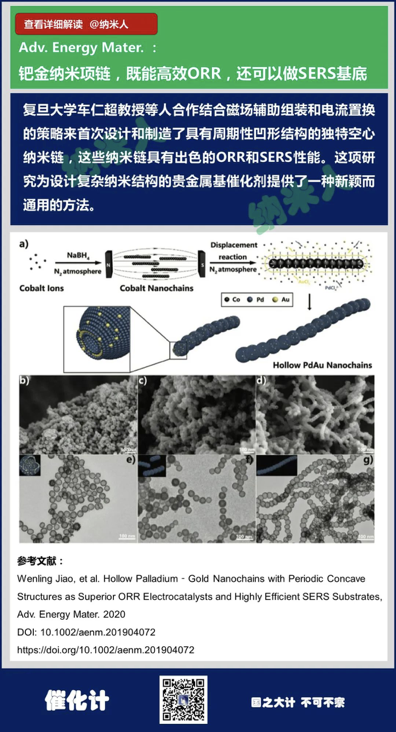
7. Wenling Jiao, et al. HollowPalladium‐Gold Nanochains with PeriodicConcave Structures as Superior ORR Electrocatalysts and Highly Efficient SERSSubstrates, Adv. Energy Mater. 2020
DOI: 10.1002/aenm.201904072
https://doi.org/10.1002/aenm.201904072
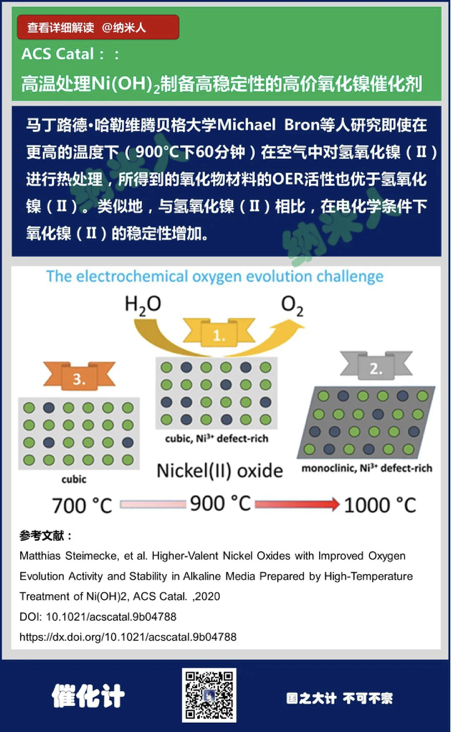
8. Matthias Steimecke, et al.Higher-Valent Nickel Oxides with Improved Oxygen Evolution Activity andStability in Alkaline Media Prepared by High-Temperature Treatment of Ni(OH)2,ACS Catal. ,2020
DOI: 10.1021/acscatal.9b04788
https://dx.doi.org/10.1021/acscatal.9b04788
9. Xing Wei, et al. HighlySelective Reduction of CO2 to C2+ Hydrocarbons at Cu/Polyaniline Interfaces.2020. ACS catalysis
DOI: 10.1021/acscatal.0c00049
https://pubs.acs.org/doi/10.1021/acscatal.0c00049
10. Mingzhen Hu, et al.Template-free Synthesis of Mesoporous and Crystalline Transition Metal OxideNanoplates with Abundant Surface Defects. Matter, 2020, 2, 1–16.
DOI: 10.1016/j.matt.2020.02.002
https://www.sciencedirect.com/science/article/pii/S2590238520300643
11. Wei Wang, et al.Quatermetallic Pt-based ultrathin nanowires intensified by Rh enable highlyactive and robust electrocatalysts for methanol oxidation. Nano Energy, 2020.
DOI: 10.1016/j.nanoen.2020.104623
https://doi.org/10.1016/j.nanoen.2020.104623
12. Yi Li, et al. TernaryPtIrNi Catalysts for Efficient Electrochemical Ammonia Oxidation. ACS Catal.2020,
DOI: 10.1021/acscatal.9b04670
https://pubs.acs.org/doi/abs/10.1021/acscatal.9b04670
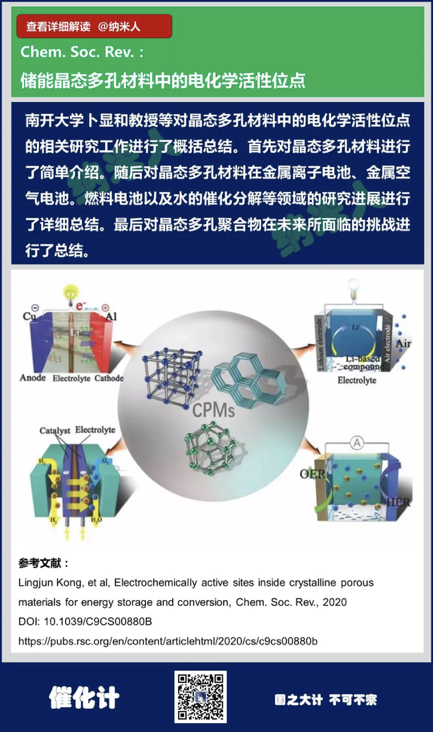
13. Lingjun Kong, et al,Electrochemically active sites inside crystalline porous materials for energystorage and conversion, Chem. Soc. Rev., 2020
DOI: 10.1039/C9CS00880B
https://pubs.rsc.org/en/content/articlehtml/2020/cs/c9cs00880b
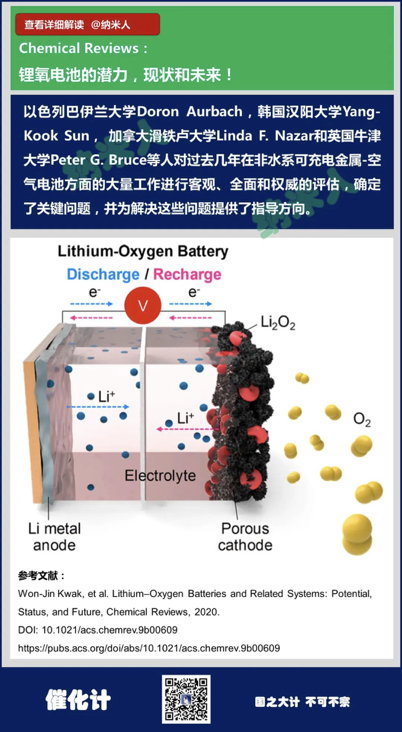
14. Won-Jin Kwak, et al.Lithium–Oxygen Batteries and Related Systems: Potential, Status, and Future,Chemical Reviews, 2020.
DOI:10.1021/acs.chemrev.9b00609
https://pubs.acs.org/doi/abs/10.1021/acs.chemrev.9b00609
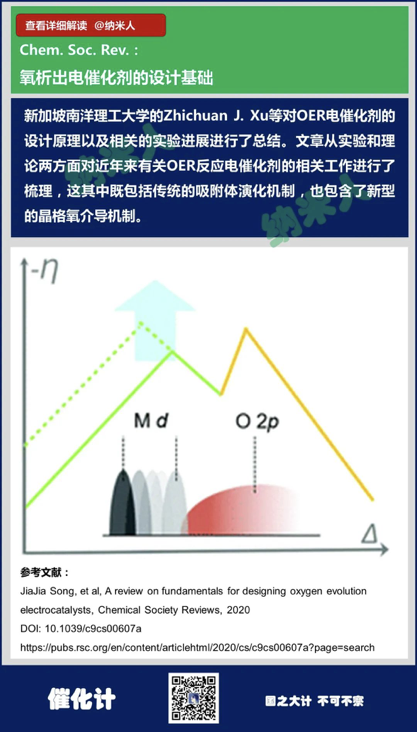
15. JiaJia Song, et al, Areview on fundamentals for designing oxygen evolution electrocatalysts, ChemicalSociety Reviews, 2020
DOI: 10.1039/c9cs00607a
https://pubs.rsc.org/en/content/articlehtml/2020/cs/c9cs00607a?page=search
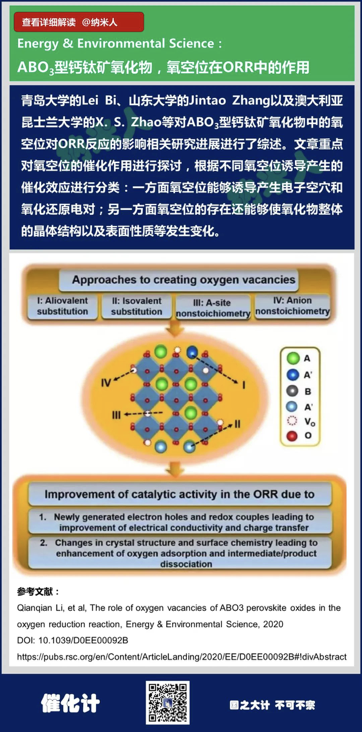
16. Qianqian Li, et al, Therole of oxygen vacancies of ABO3 perovskite oxides in the oxygen reductionreaction, Energy & Environmental Science, 2020
DOI: 10.1039/D0EE00092B
https://pubs.rsc.org/en/Content/ArticleLanding/2020/EE/D0EE00092B#!divAbstract
17. Liangxu Lin, et al.Engineered 2D Transition Metal Dichalcogenides—A Vision of Viable HydrogenEvolution Reaction Catalysis, Adv. Energy Mater. 2020
DOI: 10.1002/aenm.201903870
https://doi.org/10.1002/aenm.201903870












