
過(guò)氧化氫作為重要的化工原料,在能源、環(huán)境、醫(yī)療領(lǐng)域均中發(fā)揮著重要的作用。在眾多過(guò)氧化氫合成技術(shù)中,以氧氣與水作為原料,太陽(yáng)光作為能量源的人工光合成技術(shù)顯示出巨大的環(huán)保優(yōu)勢(shì)以及經(jīng)濟(jì)價(jià)值,因此受到越來(lái)越多科研工作者的重視。在人工光合成雙氧水的過(guò)程中,粉末光催化劑首先受光激發(fā)而產(chǎn)生電子空穴對(duì)。在光催化劑內(nèi)建電場(chǎng)的作用下,這些光生電子空穴對(duì)會(huì)被部分分離,遷移到光催化劑表面而進(jìn)行反應(yīng)。然而目前的體系中,大部分光生電子空穴對(duì)在被分離前進(jìn)行了復(fù)合,導(dǎo)致當(dāng)前人工光合成過(guò)氧化氫的太陽(yáng)能轉(zhuǎn)化效率依然低下而無(wú)法滿(mǎn)足工業(yè)化應(yīng)用。因此在該研究課題上亟需開(kāi)發(fā)有效增強(qiáng)電子空穴分離效率的策略。然而在反應(yīng)中,粉末光催化劑的表面在納米尺度內(nèi)同時(shí)富集電子和空穴,這對(duì)電荷富集位點(diǎn)的精確調(diào)控造成了困難。近日,浙江大學(xué)環(huán)資學(xué)院褚馳恒團(tuán)隊(duì)基于光電化學(xué)-半導(dǎo)體物理理論,提出了一個(gè)通過(guò)調(diào)節(jié)光催化劑表面能帶結(jié)構(gòu)來(lái)增強(qiáng)電荷分離的普適性策略。以通過(guò)BiVO4進(jìn)行光催化合成雙氧水為例,具有(銀/鈀)核/殼結(jié)構(gòu)納米顆粒(Ag/Pd)和氧化鈷顆粒(CoOx)分別被選擇性地負(fù)載在晶面化的BiVO4顆粒的還原性和氧化性晶面上。相比單獨(dú)的Pd作為還原端助催化劑,Ag/Pd能夠有效降低還原性晶面的肖特基勢(shì)壘,—同時(shí)提供足夠的表面反應(yīng)活性點(diǎn)位生成雙氧水;CoOx則快速捕捉氧化性晶面上的空穴的同時(shí),提供表面反應(yīng)活性點(diǎn)位生成氧氣。在保證表面催化性能的前提下,通過(guò)調(diào)節(jié)還原性和氧化性晶面間的能帶結(jié)構(gòu),,有效地增強(qiáng)了BiVO4中光生電子空穴對(duì)分離效率,將太陽(yáng)能轉(zhuǎn)化過(guò)氧化氫效率提高到0.73 %,突破了無(wú)機(jī)材料合成過(guò)氧化氫記錄。上述工作近日發(fā)表在Nature Communications上發(fā)表。1) BiVO4能帶結(jié)構(gòu)的選擇性調(diào)控。作者通過(guò)光沉積的方式選擇性調(diào)控BiVO4不同晶面的內(nèi)建電場(chǎng),如同電化學(xué)沉積一樣實(shí)現(xiàn)氧化與還原位點(diǎn)的能帶結(jié)構(gòu)的精準(zhǔn)調(diào)控。利用晶面化BiVO4的光生電子與空穴會(huì)選擇性地富集在還原性{010}與氧化性{110}晶面上,作者以AgNO3與PdCl42-分別作為Ag與Pd的前驅(qū)體,成功通過(guò)兩步光化學(xué)沉積將金屬Ag與Pd選擇性的沉積在{010}晶面上形成核/殼結(jié)構(gòu)的Ag/Pd納米顆粒。同時(shí),以Co(NO3)2為前驅(qū)體在{110}晶面上被負(fù)載CoOx(Fig.1a)。TEM-EDS的線掃與面掃確認(rèn)了Ag/Pd和CoOx納米顆粒分別選擇性地負(fù)載在BiVO4的{010}和{110}晶面上(Fig.1b-d)晶面上。BiVO4中的內(nèi)建電場(chǎng)受到與其接觸的金屬影響,功函數(shù)高的金屬會(huì)形成高肖特基勢(shì)壘從而阻礙電子往界面遷移,反之則利于電子遷移。由于Ag的功函數(shù)較低,產(chǎn)生的肖特基勢(shì)壘也較低(Fig. 1e),從而促進(jìn)電子往{010}面,空穴往{110}面進(jìn)行遷移(Fig. 1f)。光生電子空穴最終會(huì)分別移動(dòng)到Pd和CoOx上,進(jìn)行雙氧水生成和氧氣生成的反應(yīng)。
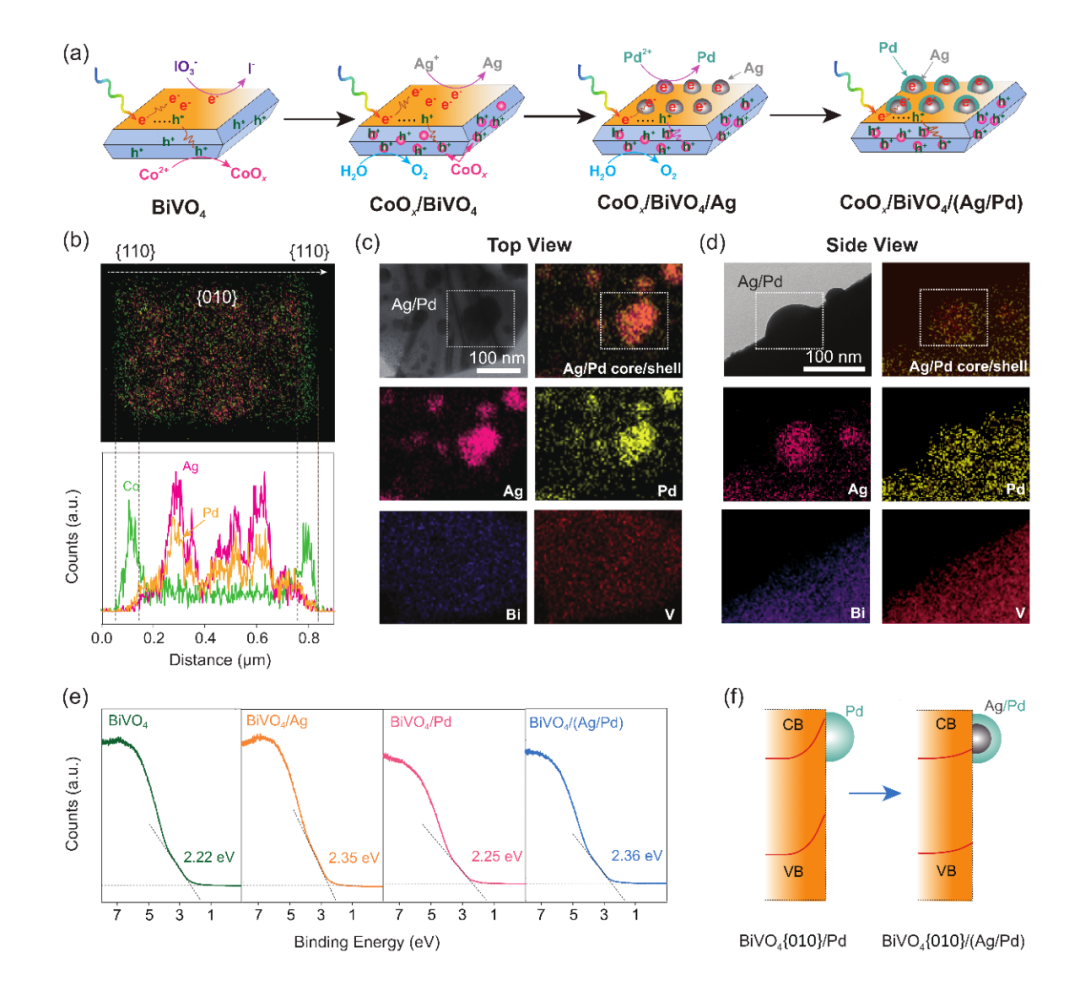
Fig. 1. Facet-selective loading of cocatalysts on BiVO4 and interfacial energetics tuning with Ag/Pd core/shell cocatalyst. (a) Stepwise and facet-selective photodeposition of Co, Ag and Pd on BiVO4. (b) Energy-dispersive X-ray spectroscopy (EDS) elemental mapping and line profile along with the white arrow of CoOx/BiVO4/(Ag/Pd). We increased Co (2 wt%), Ag (1 wt%), and Pd (1 wt%) loadings for more clear observation. (c)-(d) Scanning transmission electron microscopy (STEM)-EDS elemental mapping of Ag/Pd particles loaded on BiVO4.We increased Ag (1 wt%) and Pd (1 wt%) loadings for more clear observation. (e) Ultraviolet photoelectron spectroscopy (UPS) spectra of BiVO4, BiVO4/Ag, BiVO4/Pd and BiVO4/(Ag/Pd). (f) Schematic illustration of {010} reduction facet interfacial energetics tuning through Ag/Pd core/shell cocatalyst construction on BiVO4.為了考察能帶結(jié)構(gòu)變化與光催化性能的關(guān)系,接下來(lái)作者對(duì)比了不同催化劑的H2O2產(chǎn)出效果進(jìn)行評(píng)估。作者之前的研究表明,CoOx/BiVO4/Pd具備較為優(yōu)越光催化生成雙氧水性能。新開(kāi)發(fā)的CoOx/BiVO4/(Ag/Pd)的活性則是CoOx/BiVO4/Pd的2.1倍。由于二者的表面反應(yīng)都在Pd和CoOx上進(jìn)行,表面催化性能十分相近,活性的提升歸功于Ag增強(qiáng)電荷分離效率。UPS測(cè)試顯示Ag拉開(kāi)了價(jià)帶(Evb)到費(fèi)米能級(jí)(Efermi)的能級(jí)差值,表明低功函的Ag降低了BiVO4還原性{010}晶面上的肖特基勢(shì)壘,進(jìn)而增強(qiáng)光生電荷分離。在最優(yōu)狀態(tài)下,CoOx/BiVO4:Mo/(Ag/Pd)的全光譜量子產(chǎn)率達(dá)到3 %,其中420 nm處的量子產(chǎn)率超過(guò)了12 %,而太陽(yáng)能轉(zhuǎn)化效率達(dá)到了0.73 %,遠(yuǎn)高于現(xiàn)階段報(bào)道的無(wú)機(jī)光催化劑。
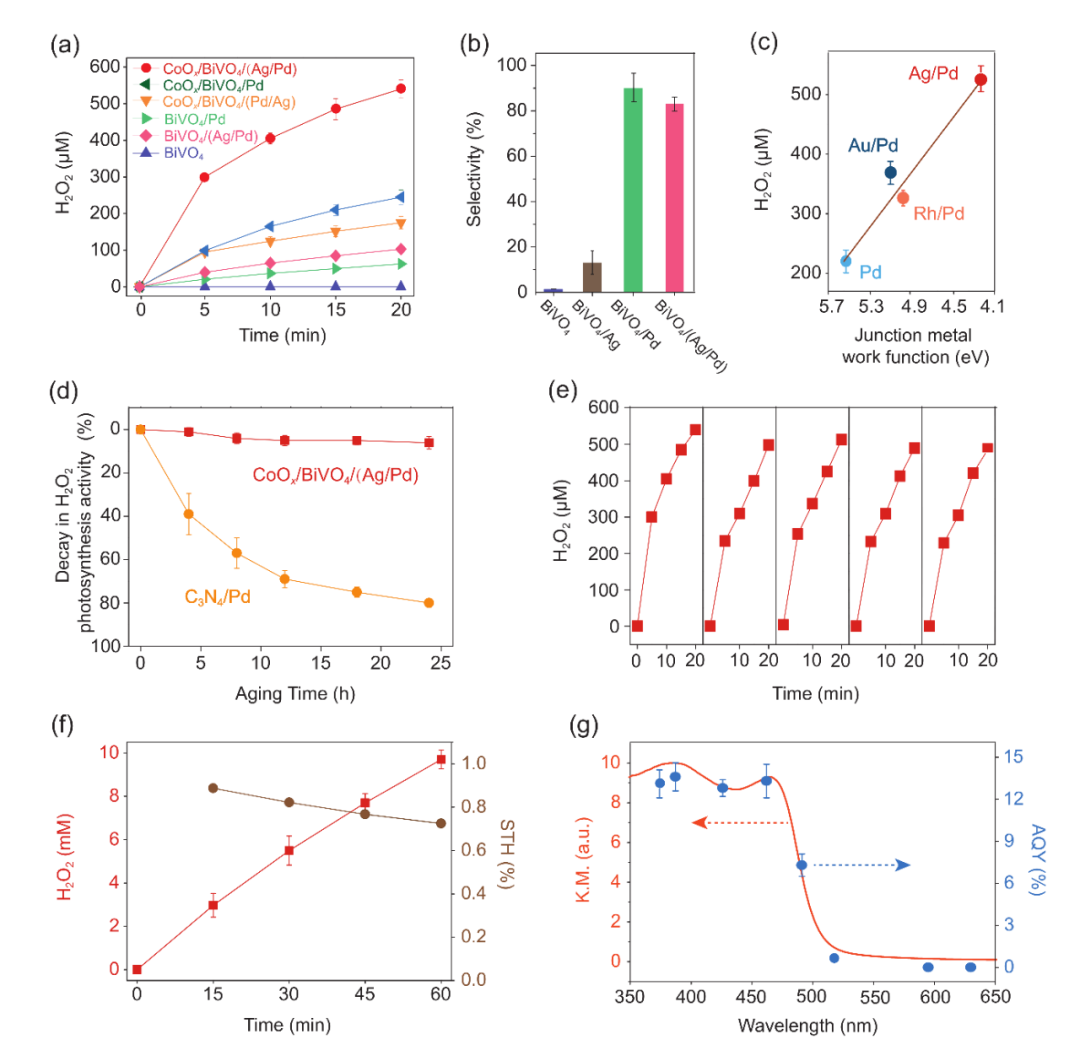
Fig. 2. Overall H2O2 photosynthesis activities. (a) Time courses of photocatalytic H2O2 generation. Reaction conditions: photocatalyst, 1 mg/mL; 50 ml DI water saturated with O2; light source, LED visible light, 300 mW cm?2, λ > 400 nm. (b) Selectivity of H2O2 production for BiVO4, BiVO4/Ag, BiVO4/Pd, and BiVO4/(Ag/Pd). Reaction conditions: photocatalyst, 1 mg/ml; 50 mL DI water with 0.1 M H3BO3and 0.075 M ScCl3 saturated with O2 (pH 6.8), 10 v/v% methanol as electron donor; light source, LED visible light, 100 mW/cm2, λ > 400 nm. H2O2 selectivity is defined as the ratio of electrons utilized for H2O2 synthesis to the total number of electrons consumed (i.e., electrons donated by methanol). (c) Preparation of various core/shell cocatalyst and correlation between H2O2photosynthesis performance and core junction metal work function. Reaction conditions: photocatalyst, 1 mg/ml; 50 mL DI water saturated with O2(pH 6.8); light source, LED visible light, 300 mW/cm2, λ > 400 nm; reaction time, 20 min. (d) Decay in H2O2 photosynthesis activity of CoOx/BiVO4/(Ag/Pd) and C3N4/Pd under ?OH-rich conditions. Aging conditions: photocatalyst, 1 mg/mL; 50 ml DI water with 25 mM H2O2; light source, 254 nm ultraviolet radiation light; ?OH was generated via the reaction H2O2 + hν→2?OH. (e) Repetitive use of CoOx/BiVO4/(Ag/Pd) for H2O2 photosynthesis. Reaction conditions: photocatalyst amount, 1 mg/ml; 50 ml DI water saturated with O2; light source, LED visible light, 300 mW cm?2, λ > 400 nm. (f) Time courses of photocatalytic H2O2 generation over CoOx/Mo:BiVO4/(Ag/Pd) and the corresponding STH efficiency. Reaction conditions: photocatalyst, 10 mg; photocatalyst, 1 mg/ml; 10 mL DI water with 0.1 M H3BO3and 0.075 M ScCl3 saturated with O2 (pH 6.8); light source, xenon lamp solar simulator, 100 mW cm?2, AM 1.5 G; irradiation area, 4.5 cm?2. (g) Apparent quantum yield (AQY) of H2O2photosynthesis over CoOx/Mo:BiVO4/(Ag/Pd) as a function of the incident light wavelength. Reaction conditions: photocatalyst, 10 mg; photocatalyst, 1 mg/ml; 10 mL DI water with 0.1 M H3BO3and 0.075 M ScCl3 saturated with O2 (pH 6.8); light source, monochromatic LED light.在活性評(píng)估之后,作者進(jìn)一步使用瞬態(tài)吸收光譜追蹤空間電荷信號(hào)衰減并探查空間電荷傳輸與能帶結(jié)構(gòu)之間的關(guān)系。通過(guò)對(duì)比CoOx/BiVO4/Pd與CoOx/BiVO4(Ag/Pd)的光生載流子的瞬態(tài)吸收光譜信號(hào)衰減情況可知,CoOx/Mo:BiVO4/(Ag/Pd)的光生載流子的壽命遠(yuǎn)大CoOx/BiVO4/Pd,表明Ag/Pd核殼結(jié)構(gòu)的引入提高了空間電荷的分離能力 (圖3a 和b)。[PZ1] CoOx與Pd雖然提供了較高的表面反應(yīng)動(dòng)力學(xué)但是結(jié)合先前的工作分析,反應(yīng)的限速步仍然是空穴端的電荷傳輸,Ag/Pd納米顆粒的引入顯著改善了光生空穴的在CoOx表面的富集,同時(shí)在一定程度上提高了光生電子在Pd表面的富集,兩種電荷傳輸增益效果共同作用提升了催化效率。
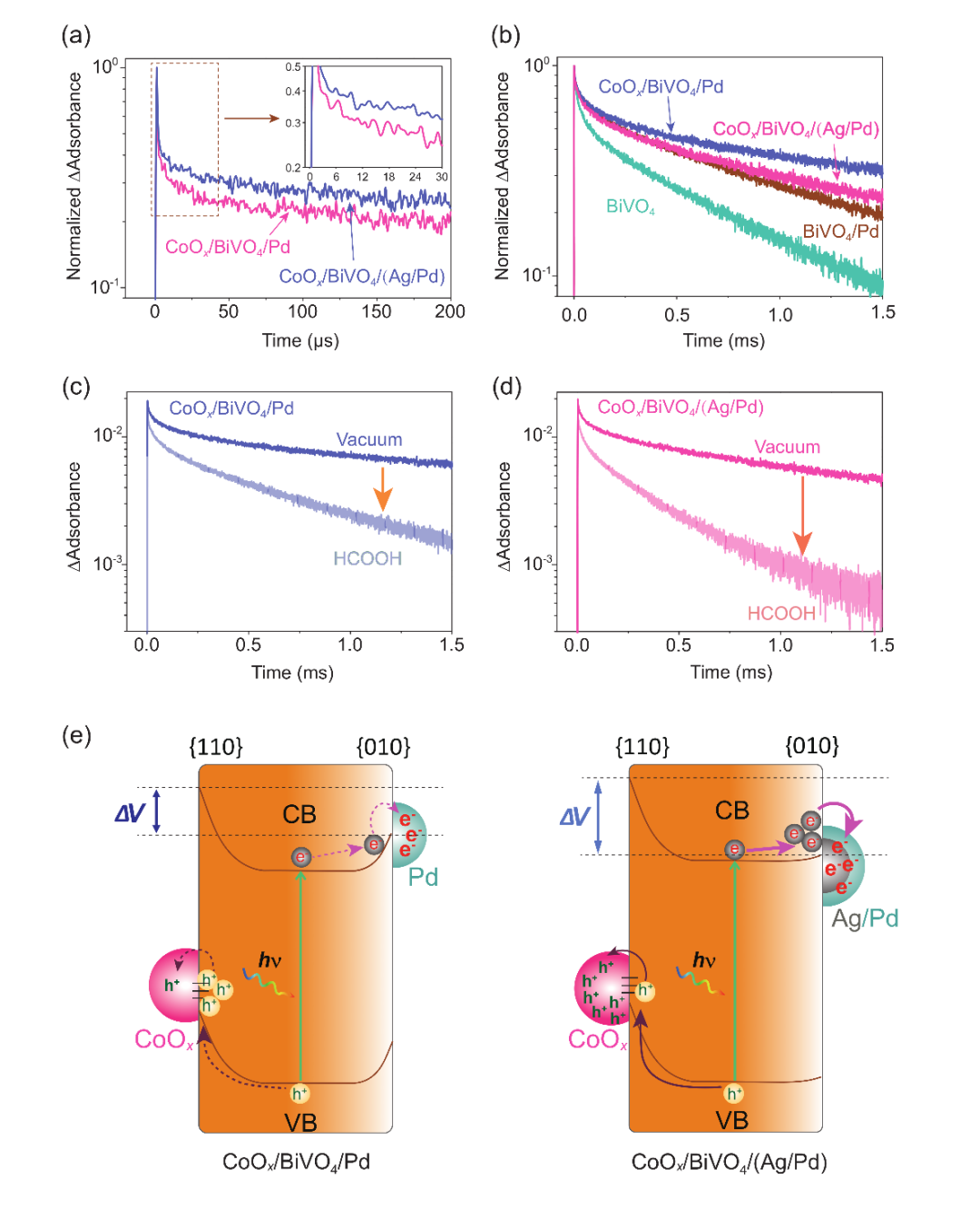
Fig. 3. Charge-carrier dynamics. (a)-(b) Transient profiles of (a) free/shallowly trapped electrons probed at 2000 nm and (b) trapped holes probed at 505 nm. Photoexcitation of the samples was performed using 470 nm laser pulses (duration: 6 ns, fluence: 3 mJ/pulse, frequency: 1 Hz). Measurements were carried out in vacuum (base pressure: ~ 10?5Torr). (c)-(d) The transient profiles of trapped holes probed at 505 nm for (c) CoOx/BiVO4/Pd and (d) CoOx/BiVO4/(Ag/Pd) in vacuum and in the presence of HCOOH vapor. Photoexcitation of the samples was performed using 470 nm laser pulses (duration: 6 ns, fluence: 3 mJ/pulse, frequency: 1 Hz). Measurements were carried out in vacuum or in the presence of 20 Torr HCOOH. (e) Schematic illustration of the charge-separation process enhanced by surface energetics tuning.作者進(jìn)一步在COMSOL中搭建了BiVO4顆粒的3D模型,對(duì)電荷分離過(guò)程進(jìn)行分析。粉體光催化劑集成了氧化與還原活性位點(diǎn),分別對(duì)應(yīng)了電化學(xué)的陽(yáng)極與陰極,作者因此從電化學(xué)角度解析空間電荷分離以及Ag/Pd的能帶結(jié)構(gòu)調(diào)控設(shè)計(jì),從電勢(shì)差的角度解析晶面控制與核殼結(jié)構(gòu)相結(jié)合的空間電荷分離路徑設(shè)計(jì),展示了如何產(chǎn)生更大的電壓。通過(guò)有限元模擬結(jié)合電化學(xué)測(cè)試模擬了CoOx/BiVO4/Pd與CoOx/BiVO4/(Ag/Pd)的電荷傳輸過(guò)程。模擬結(jié)果表明,Ag/Pd核殼結(jié)構(gòu)的引入提升空間電荷分離效率,對(duì)于單一BiVO4顆粒來(lái)說(shuō)大幅度增強(qiáng)了其{010}與{110}晶面之間的光電壓,在{010}與{110}晶面上分別富集了更多的電子與空穴進(jìn)而促進(jìn)Pd上的雙氧水生成和CoOx上的氧氣生成反應(yīng),最終提高H2O2的全合成效率。
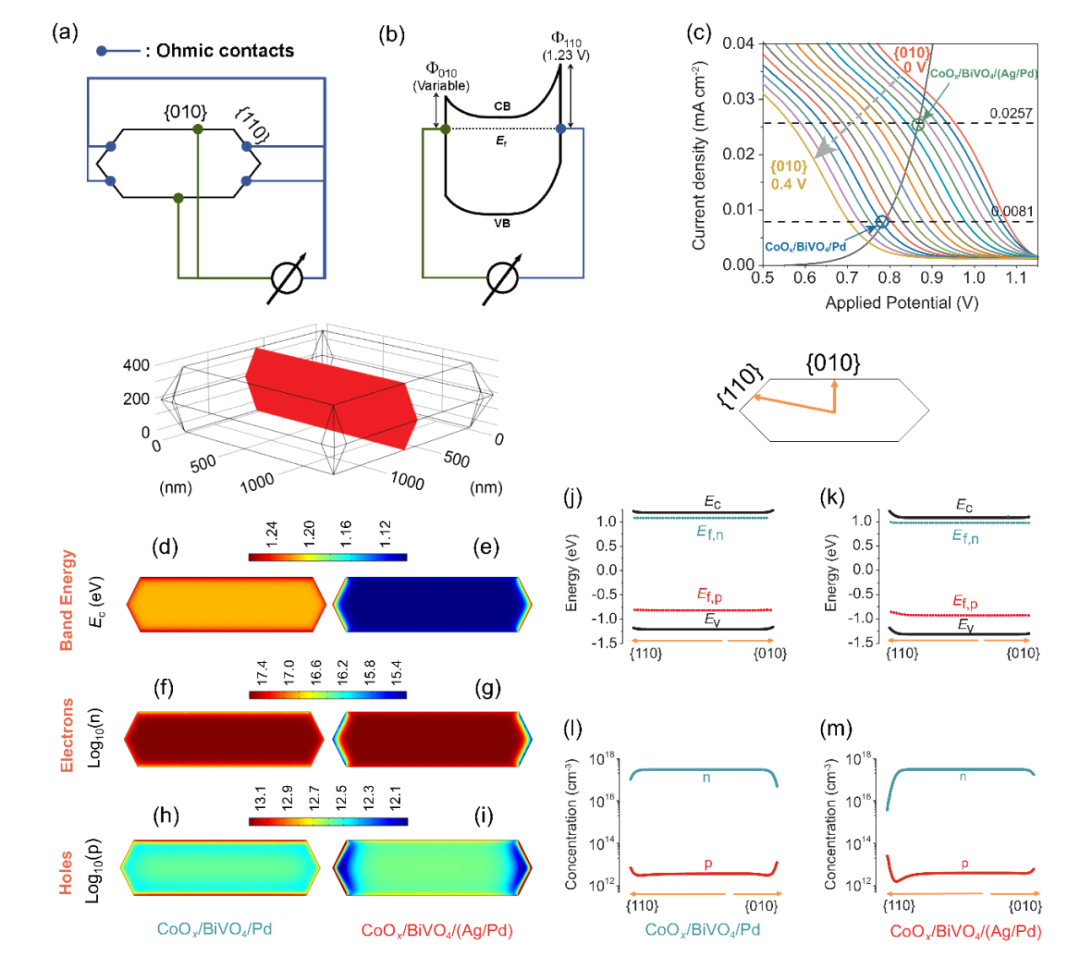
Fig. 4. Simulations of photocarrier distributions. (a)-(b) Schematic model and band diagram of BiVO4as a solar cell. (c) Current density vs. applied potential with barrier heights at cathodic sites (F010) varied from 0 to 0.4 V (grey dotted arrow). The barrier height of the anodic side was fixed at 1.23 V. Two dash lines labeled with 0.0081 and 0.0257 mA cm-2 were used to indicate the photocurrent densities converted from experimental H2O2 generation rates of CoOx/BiVO4/Pd and CoOx/BiVO4/(Ag/Pd), respectively. The operating conditions of CoOx/BiVO4/Pd and CoOx/BiVO4/(Ag/Pd) were marked with two void circles. Their detailed optoelectronic properties are presented in the following figures. (d)-(i) 2D cross-sectional plots of the optoelectronic properties of a BiVO4 particle, including conduction band energy (eV) (d)-(e), electron concentration (f)-(g), and hole concentration (h)-(i). (j)-(m) 1D plots of energy band diagram (j)-(k) and mobile charge carrier density (l)-(m).5)人工光合作用中能帶調(diào)控的通用設(shè)計(jì)思路。基于以上結(jié)果(圖1-圖4),我們提出了一種通用的能帶調(diào)控方法(圖5c) : (i) 應(yīng)用晶面工程將表面能帶結(jié)構(gòu)分離實(shí)現(xiàn)電子和空穴的空間分離; (ii) 選擇性地在氧化還原所需晶面上沉積具有相應(yīng)功能的金屬/金屬氧化物物種來(lái)調(diào)節(jié)電荷分離的表面能帶結(jié)構(gòu); 以及 (iii) 選擇性地在金屬/金屬氧化物物種上沉積助催化劑來(lái)調(diào)節(jié)表面反應(yīng)動(dòng)力學(xué)。
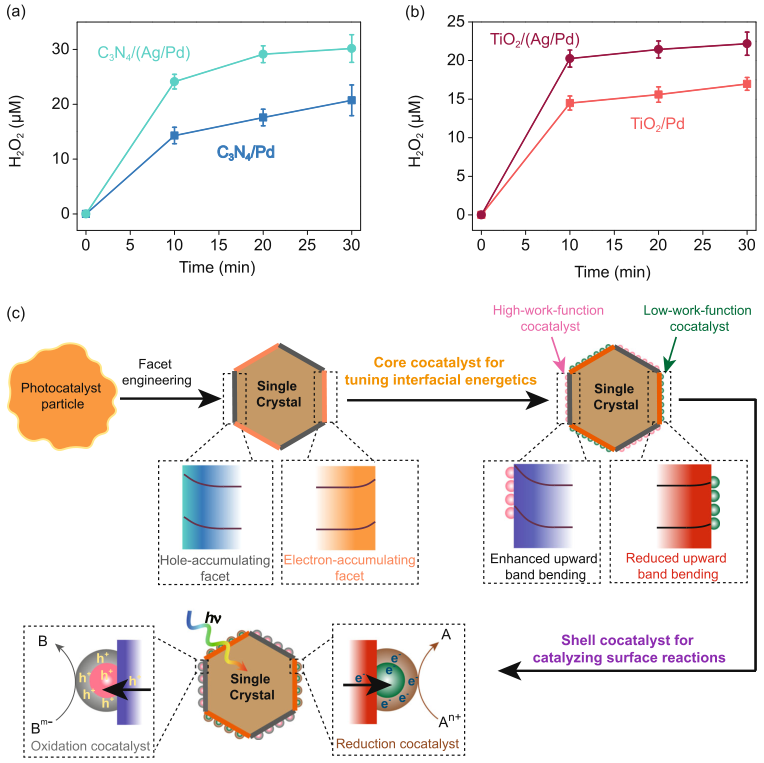
Fig. 5.Generality of interfacial-energetics-tuning strategy for enhancing artificial photosynthesis. Time courses of photocatalytic H2O2generation by (a) C3N4/Pd and C3N4/(Ag/Pd) and (b) TiO2/Pd and TiO2/(Ag/Pd). Reaction conditions: photocatalyst, 1 mg/mL; 50 ml DI water saturated with O2; light source, LED visible light (300 mW cm?2, λ > 400 nm) for C3N4and UVA light (λ = 365 nm) for TiO2. (c) A general approach for effective interfacial-energetics-tuning and enhanced artificial photosynthesis.本研究利用晶面控制手段精確調(diào)節(jié)光催化劑表面能帶結(jié)構(gòu)進(jìn)而增強(qiáng)光生電荷分離效率,將能帶彎曲調(diào)控手段從光電催化領(lǐng)域引入光催化領(lǐng)域,大幅度地提升了光催化劑的活性。將該策略應(yīng)用在光催化合成過(guò)氧化氫領(lǐng)域中,顯著提高了太陽(yáng)能到過(guò)氧化氫化學(xué)能的轉(zhuǎn)化效率,為過(guò)氧化氫綠色合成提供了新的思路。考慮到人工光合成反應(yīng)的共同性,該策略也可能適用于光催化分解水以及二氧化碳還原。

褚馳恒:浙江大學(xué)百人計(jì)劃研究員,從事環(huán)境微界面化學(xué)與污染控制方向研究。先后在北京大學(xué)、東京大學(xué)、蘇黎世聯(lián)邦理工學(xué)院獲得學(xué)士、碩士、博士學(xué)位,2016年博士畢業(yè)后在耶魯大學(xué)從事博士后研究,2019年入職浙江大學(xué)資環(huán)學(xué)院。課題組主頁(yè)https://person.zju.edu.cn/chihengchu

潘振華:日本中央大學(xué)助教。多年來(lái)從事光催化反應(yīng)以及相關(guān)半導(dǎo)體物理的研究,然而鮮有建樹(shù)。因沉迷游戲而工作懶散拖沓,備受詬病卻屢教不改。

劉添:中國(guó)科學(xué)技術(shù)大學(xué)蘇州高等研究院,特任副研究員,2020年博士畢業(yè)于湖南大學(xué)化學(xué)化工學(xué)院,2022年從浙江大學(xué)環(huán)境與資源學(xué)院博士后出站,主要從事環(huán)境功能材料設(shè)計(jì)與應(yīng)用方面的研究。


















